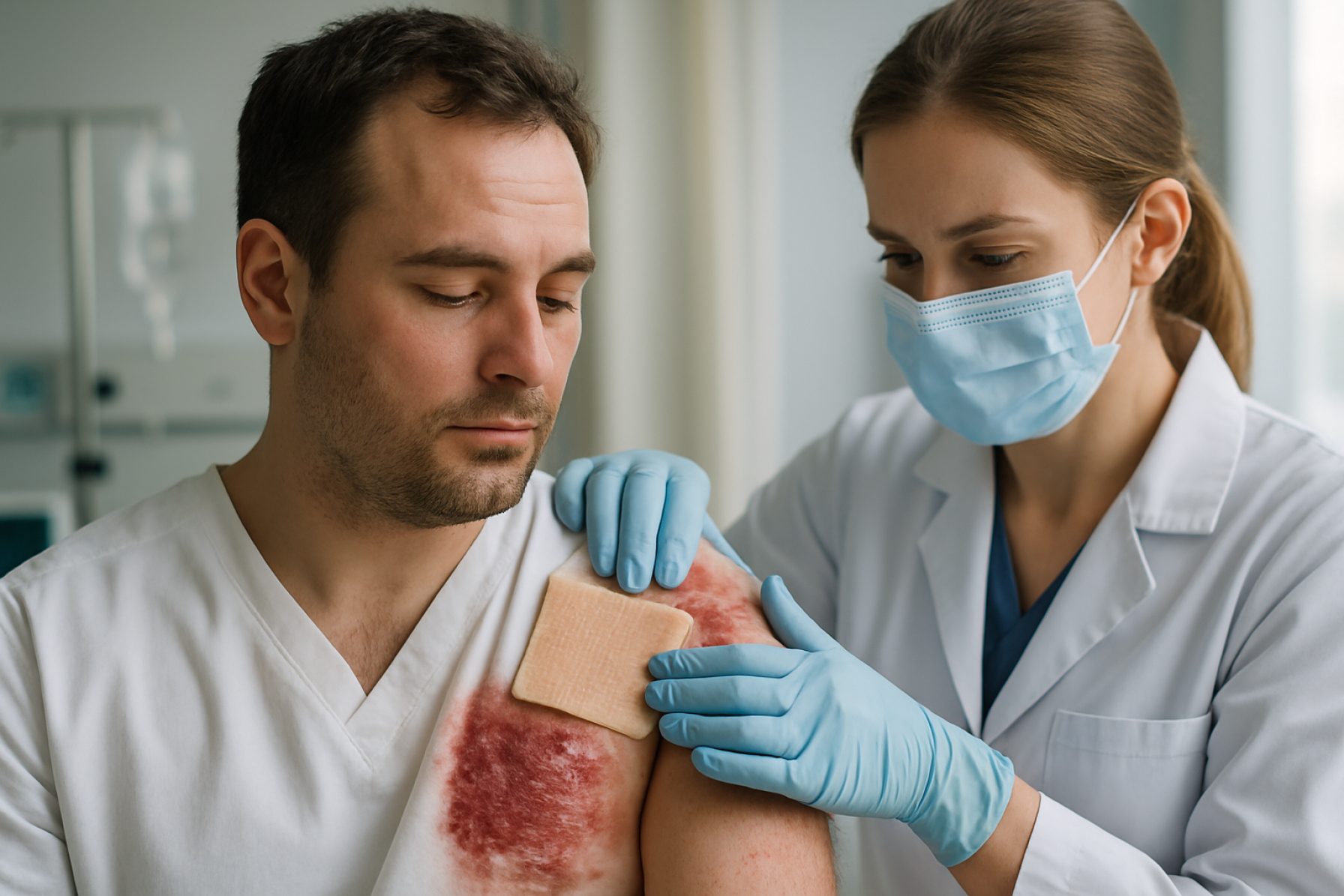
Skin Xenotransplantation Technologies in 2025: Pioneering a New Era for Severe Burn Treatment and Tissue Engineering. Explore the Innovations, Market Forces, and Regulatory Shifts Shaping the Next Five Years.
- Executive Summary: The State of Skin Xenotransplantation in 2025
- Market Size, Growth Forecasts, and Key Drivers (2025–2030)
- Technological Innovations: From Genetically Modified Pigs to Bioengineered Grafts
- Leading Companies and Research Institutions: Profiles and Strategic Initiatives
- Clinical Trials and Real-World Applications: Progress and Outcomes
- Regulatory Landscape: Approvals, Guidelines, and Global Variations
- Ethical, Safety, and Immunological Considerations in Xenotransplantation
- Supply Chain, Manufacturing, and Scalability Challenges
- Competitive Landscape: Allografts, Autografts, and Synthetic Alternatives
- Future Outlook: Disruptive Trends, Investment Hotspots, and Long-Term Impact
- Sources & References
Executive Summary: The State of Skin Xenotransplantation in 2025
Skin xenotransplantation—the transplantation of skin from non-human species to humans—has advanced rapidly in recent years, driven by urgent clinical needs and breakthroughs in genetic engineering. As of 2025, the field is transitioning from experimental and compassionate-use cases toward early-stage clinical adoption, particularly for severe burn victims and patients with extensive skin loss where human donor grafts are insufficient or unavailable.
The most significant progress has been made with genetically modified porcine (pig) skin, which offers a scalable and immunologically compatible alternative to human allografts. Companies such as Revivicor and eGenesis are at the forefront, leveraging advanced gene-editing technologies to remove key pig antigens that trigger human immune rejection. In 2023 and 2024, both companies reported successful preclinical trials demonstrating reduced hyperacute rejection and improved graft survival in non-human primate models. These results have paved the way for the first-in-human clinical trials, which are expected to expand in 2025 and beyond.
In parallel, Universal Skin and other emerging biotech firms are developing “universal donor” pig skin lines, further minimizing immunogenicity and the risk of zoonotic disease transmission. These efforts are supported by rigorous screening and pathogen inactivation protocols, in line with evolving regulatory guidance from agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
The clinical outlook for skin xenotransplantation in 2025 is cautiously optimistic. Early compassionate-use cases in the United States and Europe have shown that genetically engineered pig skin can provide temporary wound coverage, promote healing, and reduce infection risk, with manageable immunosuppression regimens. However, long-term engraftment and full immunological acceptance remain challenges, and most applications are currently limited to temporary coverage rather than permanent grafting.
Looking ahead, the next few years are expected to see:
- Expansion of clinical trials for genetically modified pig skin in major burn centers worldwide.
- Further refinement of gene-editing techniques to enhance graft survival and safety.
- Increased collaboration between biotech companies, academic centers, and regulatory bodies to establish standardized protocols and safety benchmarks.
- Potential commercial launch of the first approved xenogeneic skin products for clinical use, contingent on trial outcomes and regulatory review.
In summary, 2025 marks a pivotal year for skin xenotransplantation technologies, with industry leaders such as Revivicor and eGenesis driving the transition from laboratory innovation to real-world clinical solutions. The coming years will be critical in determining the scalability, safety, and acceptance of these transformative therapies.
Market Size, Growth Forecasts, and Key Drivers (2025–2030)
The global market for skin xenotransplantation technologies is poised for significant growth between 2025 and 2030, driven by advances in genetic engineering, increasing incidence of severe burns and chronic wounds, and a persistent shortage of human donor skin. As of 2025, the sector remains in a pre-commercial or early commercial phase, with several companies and research consortia advancing genetically modified porcine skin grafts toward clinical application.
Key players in this space include Revivicor, a subsidiary of United Therapeutics, which has pioneered the development of genetically engineered pigs for organ and tissue transplantation. Their GalSafe™ pigs, designed to eliminate the alpha-gal sugar responsible for hyperacute rejection, have received FDA approval for food and medical use, laying the groundwork for clinical-grade xenografts. United Therapeutics continues to invest in scaling up production and refining immunological compatibility for skin applications.
Another notable company is Xenothera, which is developing immunomodulatory solutions and has ongoing research in xenotransplantation, including skin. Additionally, eGenesis is leveraging CRISPR gene editing to create porcine tissues with multiple genetic modifications to reduce rejection and pathogen transmission risks, with skin grafts as a potential application.
Market growth is underpinned by the rising global burden of burn injuries—estimated at 11 million people annually worldwide—and the increasing prevalence of chronic wounds, particularly among aging populations and those with diabetes. The limited availability of allogeneic (human) skin grafts, especially in mass casualty or military settings, further accelerates demand for alternative solutions such as xenografts.
From 2025 onward, the market is expected to transition from experimental and compassionate-use cases to early commercial adoption, particularly in regions with advanced regulatory frameworks and established biomanufacturing capabilities. The U.S. FDA’s progressive stance on xenotransplantation, as evidenced by recent approvals, is likely to catalyze similar regulatory pathways in Europe and Asia-Pacific.
Key growth drivers for 2025–2030 include:
- Advancements in gene editing and pathogen screening, improving graft safety and compatibility.
- Strategic partnerships between biotech firms, academic centers, and healthcare providers to accelerate clinical trials and commercialization.
- Government and defense sector interest in stockpiling xenograft skin for emergency preparedness.
- Increasing investment in biomanufacturing infrastructure to enable scalable production of clinical-grade porcine skin.
While the market remains nascent in 2025, projections indicate robust growth potential as clinical efficacy and safety are demonstrated, regulatory approvals expand, and manufacturing capacity scales. The next five years are likely to see the first commercial xenograft skin products reach select hospitals, with broader adoption anticipated as clinical and economic benefits are validated.
Technological Innovations: From Genetically Modified Pigs to Bioengineered Grafts
Skin xenotransplantation—the transplantation of skin from non-human species to humans—has seen significant technological advances as of 2025, driven by urgent clinical needs such as severe burns and chronic wounds. The field is rapidly evolving, with a focus on genetically modified pigs and bioengineered grafts designed to overcome immunological barriers and improve graft survival.
Genetically modified pigs have become the primary source for xenogeneic skin, owing to their physiological similarities to humans and the scalability of pig breeding. Companies such as Revivicor and EGEBiotech are at the forefront, engineering pigs with multiple gene edits to reduce the expression of antigens responsible for hyperacute rejection. These modifications often include the knockout of genes encoding for alpha-gal and other xenoantigens, as well as the insertion of human complement regulatory proteins to further mitigate immune responses.
In 2023 and 2024, preclinical studies demonstrated that skin grafts from these genetically modified pigs could survive for several weeks on non-human primates without immediate rejection, a significant improvement over previous attempts. Early-stage clinical trials are anticipated in 2025, with regulatory agencies closely monitoring safety and efficacy. The U.S. Food and Drug Administration (FDA) has provided guidance for xenotransplantation protocols, and companies are working to meet these stringent requirements.
Parallel to advances in genetic engineering, bioengineered skin grafts are gaining traction. These grafts combine porcine-derived extracellular matrices with human cells or synthetic scaffolds to create hybrid constructs that promote integration and healing. Organogenesis and AVITA Medical are notable players developing such products, leveraging their expertise in regenerative medicine and wound care. Their technologies aim to provide temporary or permanent coverage for extensive wounds, reducing infection risk and improving patient outcomes.
Looking ahead, the next few years are expected to see the first-in-human trials of multi-gene-edited pig skin grafts, with a focus on safety, immunogenicity, and functional integration. Advances in gene editing tools, such as CRISPR/Cas9, are likely to accelerate the development of even more compatible donor animals. Additionally, the integration of 3D bioprinting and tissue engineering may enable the customization of grafts to match patient-specific needs, further enhancing the clinical utility of xenotransplantation technologies.
Overall, the convergence of genetic engineering, tissue engineering, and regulatory progress positions skin xenotransplantation as a promising solution for critical unmet medical needs, with 2025 poised to be a pivotal year for clinical translation and commercialization.
Leading Companies and Research Institutions: Profiles and Strategic Initiatives
Skin xenotransplantation—using animal-derived skin grafts for human wound care—has advanced rapidly, with several companies and research institutions at the forefront of translating preclinical breakthroughs into clinical and commercial realities. As of 2025, the sector is characterized by a blend of established biotechnology firms, innovative startups, and academic collaborations, all aiming to address the global shortage of human donor skin for severe burns and chronic wounds.
A global leader in xenotransplantation, Revivicor (a subsidiary of United Therapeutics Corporation) has leveraged its expertise in genetic engineering of pigs to develop porcine tissues with reduced immunogenicity. While Revivicor is best known for its work in solid organ xenotransplantation, its proprietary GalSafe pigs—engineered to lack the alpha-gal sugar responsible for hyperacute rejection—are also being explored as sources for skin grafts. The company’s ongoing collaborations with academic medical centers are expected to yield early-phase clinical data on porcine skin graft safety and efficacy in the next few years.
Another prominent player, Genopole, France’s leading biocluster for genetic and biotechnological innovation, supports several startups and research groups focused on xenotransplantation. Through its incubator and funding programs, Genopole fosters the development of genetically modified animal models and advanced tissue engineering platforms, with a particular emphasis on immunomodulation and infection control for skin grafts.
In Asia, Sinogene Biotechnology has expanded its portfolio from animal cloning to the development of genetically engineered pigs for biomedical applications, including skin xenografts. The company’s research division is actively collaborating with Chinese hospitals to initiate clinical trials of porcine skin for burn patients, with regulatory submissions anticipated by 2026.
Academic institutions remain pivotal in advancing the field. The Massachusetts General Hospital (MGH) Transplant Center, in partnership with biotechnology firms, is conducting preclinical studies on immunosuppressive regimens and gene-edited porcine skin. MGH’s research is expected to inform the design of first-in-human trials in the United States, potentially commencing within the next two to three years.
Looking ahead, strategic initiatives across these organizations focus on refining genetic modifications to minimize rejection, scaling up pathogen-free animal husbandry, and navigating evolving regulatory frameworks. The next few years will likely see the first controlled clinical trials of gene-edited porcine skin, with the potential to transform burn care and wound management globally.
Clinical Trials and Real-World Applications: Progress and Outcomes
Skin xenotransplantation—the transplantation of skin from non-human species, primarily genetically modified pigs, to humans—has advanced rapidly in recent years, with 2025 marking a pivotal period for clinical translation and real-world application. The technology addresses critical shortages in autologous and allogeneic skin grafts for severe burn victims and patients with extensive skin loss, offering a potential lifeline where human donor skin is unavailable.
In 2024 and 2025, several early-phase clinical trials have been initiated or are ongoing, focusing on the safety, immunogenicity, and efficacy of porcine-derived skin grafts. Notably, Xenothera, a French biotechnology company, has been at the forefront, leveraging its expertise in immunomodulation and genetic engineering to develop porcine skin grafts with reduced immunogenicity. Their preclinical studies demonstrated promising results in minimizing acute rejection, and in 2025, the company is expected to report interim data from its first-in-human trials.
Another key player, Revivicor (a subsidiary of United Therapeutics), has developed multi-gene-edited pigs whose tissues are designed to be more compatible with the human immune system. In 2024, Revivicor’s porcine skin grafts entered compassionate use protocols in select U.S. burn centers, with early outcomes indicating improved graft survival and reduced need for immunosuppression compared to previous generations of xenografts. The company is collaborating with academic medical centers to launch formal Phase I/II trials in 2025, focusing on both acute burn care and chronic wound management.
In Asia, Sinogene, a Chinese biotechnology firm specializing in animal genetic engineering, has announced the development of gene-edited pigs for skin xenotransplantation. In 2025, Sinogene is expected to initiate pilot clinical studies in collaboration with major Chinese hospitals, aiming to address the high incidence of severe burns in the region.
Real-world applications remain limited to highly regulated clinical settings, with strict oversight from national health authorities. Early data from compassionate use and expanded access programs suggest that genetically modified porcine skin grafts can provide effective temporary wound coverage, reduce infection rates, and facilitate healing until autologous grafting is possible. However, long-term outcomes, including chronic rejection and zoonotic risk, are still under close investigation.
Looking ahead, the next few years are expected to see the expansion of clinical trials, refinement of genetic modifications to further reduce immunogenicity, and the development of standardized protocols for xenograft application. Regulatory agencies in the U.S., Europe, and Asia are closely monitoring these developments, with the potential for conditional approvals if ongoing trials continue to demonstrate safety and efficacy. The field is poised for significant breakthroughs, with the promise of transforming burn and wound care globally.
Regulatory Landscape: Approvals, Guidelines, and Global Variations
The regulatory landscape for skin xenotransplantation technologies is rapidly evolving as scientific advances bring these products closer to clinical application. As of 2025, regulatory agencies worldwide are actively developing and refining frameworks to address the unique challenges posed by xenogeneic skin grafts, particularly those derived from genetically modified pigs. These challenges include concerns about zoonotic disease transmission, immunological compatibility, ethical considerations, and long-term safety monitoring.
In the United States, the U.S. Food and Drug Administration (FDA) has established guidelines for xenotransplantation products, requiring rigorous preclinical and clinical data to demonstrate safety and efficacy. The FDA’s Center for Biologics Evaluation and Research (CBER) oversees the regulatory pathway for these products, emphasizing the need for comprehensive risk assessments, including the potential for porcine endogenous retrovirus (PERV) transmission. In recent years, the FDA has granted Investigational New Drug (IND) approvals for early-phase clinical trials of genetically engineered porcine skin grafts, reflecting a cautious but progressive stance.
In Europe, the European Medicines Agency (EMA) regulates xenotransplantation under the Advanced Therapy Medicinal Products (ATMP) framework. The EMA requires extensive documentation on donor animal health, genetic modifications, and manufacturing processes. The agency also coordinates with national authorities to ensure harmonized oversight across member states. Notably, the EMA has issued scientific advice to several companies developing porcine skin grafts, signaling growing regulatory engagement in this field.
Asia-Pacific countries, particularly Japan and South Korea, are also advancing regulatory frameworks for xenotransplantation. Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) has published guidelines for the clinical use of xenogeneic tissues, focusing on infection control and post-transplant surveillance. South Korea’s Ministry of Food and Drug Safety (MFDS) is similarly updating its regulations to accommodate emerging xenotransplantation technologies.
Globally, there is significant variation in regulatory requirements, with some countries adopting more permissive approaches to clinical trials, while others maintain strict prohibitions on xenotransplantation. International organizations such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are working to promote harmonization of standards, but substantial differences remain.
Looking ahead, the next few years are expected to see increased regulatory clarity as more clinical data become available and as companies such as Revivicor and eGenesis advance their genetically engineered porcine skin graft programs. Regulatory agencies are likely to update guidelines to reflect new scientific insights, with a continued emphasis on patient safety, traceability, and long-term monitoring. The evolving landscape will require close collaboration between industry, regulators, and the scientific community to ensure responsible and effective translation of skin xenotransplantation technologies into clinical practice.
Ethical, Safety, and Immunological Considerations in Xenotransplantation
Skin xenotransplantation—the transplantation of skin from non-human species, primarily genetically modified pigs, to humans—has advanced rapidly in recent years, but it remains subject to significant ethical, safety, and immunological scrutiny. As of 2025, the field is transitioning from preclinical studies to early-stage clinical applications, with a focus on addressing the unique challenges posed by cross-species transplantation.
A primary ethical concern centers on the use of genetically engineered animals. Companies such as Revivicor and eGenesis are at the forefront, developing pigs with multiple gene edits to reduce immunogenicity and the risk of zoonotic disease transmission. These modifications, while promising for reducing rejection, raise questions about animal welfare, long-term ecological impacts, and the acceptability of using animal organs and tissues for human benefit. Regulatory bodies in the US, EU, and Asia are actively developing frameworks to address these issues, emphasizing transparency, animal welfare standards, and public engagement.
Safety remains a paramount concern, particularly regarding the risk of transmitting porcine endogenous retroviruses (PERVs) and other pathogens. Both Revivicor and eGenesis employ advanced gene-editing technologies, such as CRISPR, to inactivate PERV sequences in donor pigs. Early 2025 data from preclinical and compassionate-use cases suggest that these measures significantly reduce, but do not entirely eliminate, the risk of zoonosis. Ongoing surveillance and stringent screening protocols are being implemented in all clinical protocols, with oversight from national health authorities and organizations such as the International Xenotransplantation Association.
Immunological rejection remains the most significant technical barrier. The human immune system mounts a rapid and robust response to xenogeneic tissues, leading to hyperacute and delayed rejection. To address this, companies are engineering pigs to lack key antigens (such as alpha-gal) and to express human complement regulatory proteins. In 2024 and 2025, several early-phase clinical trials and compassionate-use cases have demonstrated temporary engraftment of porcine skin in burn patients, providing critical wound coverage while awaiting autografting. However, long-term survival of xenografts remains elusive, and immunosuppressive regimens carry their own risks.
Looking ahead, the next few years will likely see expanded clinical trials, improved genetic engineering of donor animals, and the development of more targeted immunomodulatory therapies. The field is also moving toward greater international harmonization of ethical and safety standards, with industry leaders such as Revivicor and eGenesis collaborating with regulatory agencies and bioethics committees to ensure responsible progress. The outlook for skin xenotransplantation is cautiously optimistic, with the potential to address critical shortages in skin grafts for severe burns, provided that ethical, safety, and immunological challenges continue to be rigorously addressed.
Supply Chain, Manufacturing, and Scalability Challenges
Skin xenotransplantation technologies, which involve the transplantation of skin from non-human sources (primarily genetically modified pigs) to humans, are advancing rapidly in 2025. However, the sector faces significant supply chain, manufacturing, and scalability challenges as it moves from experimental and compassionate-use cases toward broader clinical application.
A primary challenge is the reliable and ethical sourcing of donor animals. Companies such as Revivicor (a subsidiary of United Therapeutics) have developed genetically engineered pigs designed to minimize immune rejection and zoonotic risks. These pigs require highly controlled breeding, housing, and monitoring environments to ensure biosecurity and genetic consistency, which increases operational complexity and cost. Scaling up herds to meet potential clinical demand, while maintaining rigorous standards, remains a bottleneck.
Manufacturing xenogeneic skin grafts involves complex bioprocessing steps, including harvesting, decellularization (if using acellular matrices), sterilization, and preservation. Each step must comply with stringent regulatory requirements for Good Manufacturing Practice (GMP). Revivicor and other emerging players are investing in dedicated biomanufacturing facilities, but the capital and expertise required to scale these operations are substantial. The need for specialized cleanroom environments and validated cold chain logistics further complicates the supply chain.
Another challenge is the coordination between animal facilities, processing plants, and clinical sites. The time-sensitive nature of living tissue products demands robust logistics and real-time tracking. Companies are exploring partnerships with established biopharmaceutical logistics providers to ensure timely and safe delivery, but the infrastructure for large-scale, cross-border distribution of xenogeneic tissues is still nascent.
Regulatory oversight adds another layer of complexity. Agencies such as the U.S. Food and Drug Administration (FDA) require comprehensive traceability from donor animal to patient, including documentation of genetic modifications, health status, and processing steps. This necessitates advanced digital tracking systems and transparent supply chain management, which many companies are still developing.
Looking ahead, the sector is expected to see incremental improvements in scalability as automation, digitalization, and supply chain integration mature. Companies like United Therapeutics (parent of Revivicor) are investing in vertically integrated models to control the entire process from animal husbandry to clinical delivery. However, widespread adoption will depend on overcoming current bottlenecks in animal supply, bioprocessing capacity, and regulatory harmonization across regions. The next few years will be critical for establishing robust, scalable supply chains that can support the transition of skin xenotransplantation from experimental therapy to routine clinical practice.
Competitive Landscape: Allografts, Autografts, and Synthetic Alternatives
The competitive landscape for skin xenotransplantation technologies in 2025 is rapidly evolving, driven by the urgent need for effective skin substitutes in severe burn care, chronic wounds, and reconstructive surgery. Traditional approaches—autografts (patient’s own skin) and allografts (donor human skin)—remain the clinical gold standards, but both face significant limitations. Autografts are constrained by donor site morbidity and limited availability in extensive injuries, while allografts carry risks of immune rejection and disease transmission, and are often in short supply.
In response, the field is witnessing a surge in innovation around xenotransplantation, particularly with porcine (pig-derived) skin, due to its physiological similarity to human skin and scalable supply. Companies such as Revivicor, a subsidiary of United Therapeutics, are at the forefront, leveraging advanced genetic engineering to produce pigs with reduced immunogenicity. Their work builds on the success of xenotransplantation in solid organs, with genetically modified pigs designed to minimize hyperacute rejection and other immune responses. In 2024, Revivicor’s porcine skin grafts entered early clinical evaluation in select burn centers, with initial results indicating improved graft survival and reduced inflammatory complications compared to conventional xenografts.
Another notable player is Genesis Biosciences, which is developing decellularized porcine dermal matrices. These products aim to provide a biocompatible scaffold that supports host cell infiltration and vascularization, while minimizing the risk of zoonotic disease transmission. Genesis Biosciences is collaborating with regulatory agencies in the US and Europe to advance these products through clinical trials, with pivotal data expected by late 2025.
Meanwhile, synthetic and biosynthetic skin substitutes continue to compete in the market, with established companies like Smith & Nephew and Organogenesis offering products such as Integra® and Dermagraft®, respectively. These alternatives provide off-the-shelf availability and eliminate the risk of animal or human disease transmission, but often lack the full biological functionality of living tissue and may be less effective in large or complex wounds.
Looking ahead, the next few years are expected to see increased convergence between xenotransplantation and advanced tissue engineering. Companies are exploring the integration of gene-edited porcine cells with bioactive scaffolds and 3D bioprinting technologies to create next-generation skin substitutes. Regulatory pathways are also evolving, with agencies such as the FDA and EMA providing new guidance for xenogeneic products, potentially accelerating market entry for safe and effective solutions. As clinical data accumulates and manufacturing scales up, skin xenotransplantation technologies are poised to become a transformative option in the competitive landscape of wound care and reconstructive medicine.
Future Outlook: Disruptive Trends, Investment Hotspots, and Long-Term Impact
Skin xenotransplantation—the transplantation of skin from non-human species to humans—stands at a pivotal juncture in 2025, driven by advances in genetic engineering, immunomodulation, and biomanufacturing. The field is primarily focused on addressing the critical shortage of human donor skin for severe burn victims and chronic wound patients. Recent years have seen a surge in investment and research, with several companies and research consortia pushing the boundaries of what is possible.
A major disruptive trend is the use of genetically modified porcine (pig) skin, engineered to reduce immunogenicity and the risk of zoonotic disease transmission. Companies such as Revivicor—a subsidiary of United Therapeutics—have pioneered the development of multi-gene-edited pigs whose tissues are less likely to be rejected by the human immune system. In 2023 and 2024, preclinical and early clinical studies demonstrated that porcine skin grafts could survive for weeks on human recipients without immediate rejection, a significant leap from previous attempts. These advances are underpinned by CRISPR and other gene-editing technologies, which allow for precise removal of antigens and insertion of human-compatible genes.
Another key player, eGenesis, is leveraging advanced gene-editing to create pigs with over 60 genetic modifications, targeting both immunological compatibility and the elimination of porcine endogenous retroviruses (PERVs). Their work is closely watched by regulatory agencies and the broader transplant community, as it sets the stage for first-in-human trials of xenogeneic skin in the near future.
Investment hotspots are emerging in North America, Europe, and East Asia, with public and private funding flowing into both established biotech firms and academic-industry partnerships. The U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) have signaled openness to accelerated pathways for xenotransplantation products, provided robust safety and efficacy data are presented. This regulatory flexibility is expected to catalyze further investment and clinical translation.
Looking ahead, the next few years will likely see the first approved clinical trials of gene-edited porcine skin for temporary wound coverage, especially in cases where human allografts are unavailable. If successful, these products could disrupt the current standard of care, reducing mortality and morbidity for burn patients worldwide. Long-term, the integration of xenotransplantation with tissue engineering—such as combining porcine scaffolds with human stem cells—may yield even more advanced skin substitutes.
The impact of these technologies extends beyond clinical outcomes. They promise to reshape supply chains, reduce healthcare costs, and open new markets for biomanufactured tissues. As ethical, regulatory, and technical challenges are addressed, skin xenotransplantation is poised to become a cornerstone of regenerative medicine by the end of the decade.
Sources & References
- Revivicor
- United Therapeutics
- Xenothera
- eGenesis
- Organogenesis
- AVITA Medical
- Genopole
- European Medicines Agency
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- Genesis Biosciences
- Smith & Nephew